Zeolitic materials have been widely applied in petro-, coal and fine chemical industries due to their excellent adsorptive and catalytic properties, which highly depend on their porous structures, chemical compositions, surface properties and crystal shape and size. Rationally design of zeolite with required morphology, especially precise assembly of rigid zeolite crystal, is crucial to improve the adsorption and catalytic performance. At present, significant progress has been made in accurately controlling zeolite with desired morphology by using multi tailed quaternary ammonium salts and/or amphiphilic surfactants as templates which induces the rational assembly of zeolite nano sheets into particles with mesoporous structure. However, many defect sites are formed on the surface of nanosheet, which lead to the poor hydrothermal stability of the formed zeolite, and the rigid framework structure and a small amount of Si-OH on the surface of perfectly crystallized zeolite brings difficulties to the assembly. Therefore, it is of great theoretical and practical value to develop an effective method for assembling perfect crystalline zeolite crystals.
Recently, the research team of Fan Weibin and Dong Mei from ICC has made great progress in the rational design and precise assembly of zeolite with special morphology by using organic and inorganic additives in the hydrothermal process. They developed a facile method to successfully synthesize ZSM-5 hollow microspheres with the assistance of disodium ethylenediaminetetraacetate dihydrate (Na2-EDTA) in a near neutral pH environment. The EDTA2- ions not only act as an effective mineralizer, but also interact with NBA+ ions and direct the formation of hollow sphere structure and shell with oriented assembly of intergrown ZSM-5 coffin-like crystals along the c axis via the confined mechanism. The ZSM-5 hollow spheres show high aromatics selectivity and excellent stability in the methanol to aromatics (MTA) reaction due to the presence of a larger mesoporous volume in the shell and a larger cavity in the core.
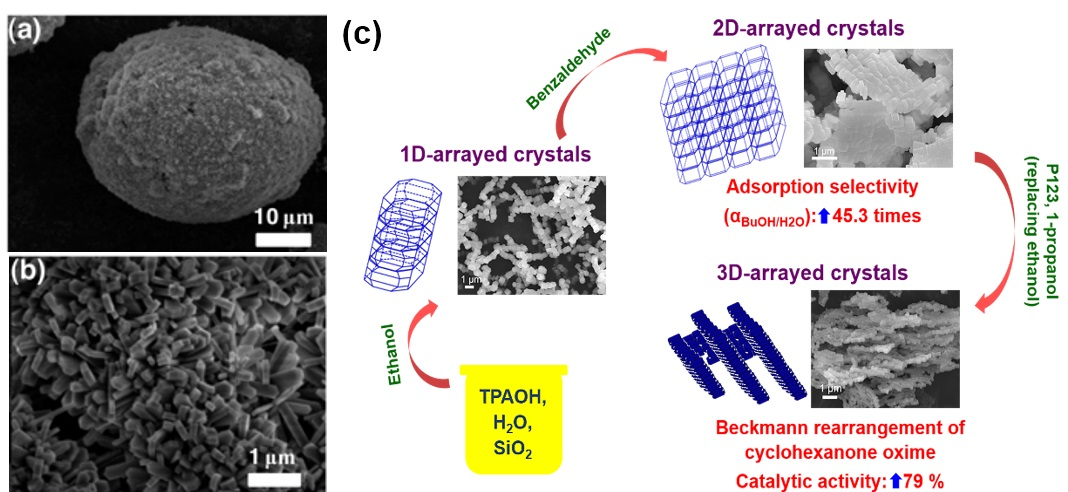
Furthermore, the research team made great success in assembling the Silicalite-1 crystals like toy Lego bricks into one-dimensional (1D), two-dimensional (2D), and three-dimensional (3D) architectures with the assistance of morphology-directing agents. A 1D architecture was formed by stacking crystals along the b axis with the assistance of ethanol that is selectively adsorbed on (100) and (001) crystal facets. Such adsorption increases the condensation energy barriers along a and c axes, but facilitates the condensation between (010) facets and the formation of 1D architecture. The addition of benzaldehyde (BAL) in the synthesis of 1D-arrayed crystals leads to a 2D architecture. In the crystallization, the preferable adsorption of BAL stabilized the (001) facet, while the pore wall surface on the (100) facet exposed to H2O molecules by significantly weakening EtOH adsorption, thus inducing the crystals alignment along both a and b axes to 2D architecture. When an amphiphilic copolymer P123 was further added in the gel along with the substitution of ethanol by 1-propanol, a 3D network was fabricated by the agglomeration and self-pillaring of the 2D Lego bricks possibly with P123 aggregates as the substrate matrix.
Owing to the increased tortuosity of pore channels in the 1D chainlike ZSM-5, the ratio of diffusion coefficient of p-xylene to that of o-xylene in the 1D zeolite reaches 3.17, which is much higher than that in the isolated ZSM-5 (1.09), demonstrating the superiority of chainlike ZSM-5 zeolites as a highly selective adsorbent or catalyst concerning guest molecules similar in dimension. Excitingly, upon alignment of crystals into 2D architectures, the adsorptive selectivity of 1-butanol (2 wt %) to water of silicalite-1 increases by 45.3 times, while into 3D networks, the catalytic activity for the Beckmann rearrangement of cyclohexanone oxime elevates by 79% along with a great enhancement of catalytic stability. In a word, this work not only provides an intriguing methodology for Lego-assembling zeolite crystals into specific architecture using morphology-directing agents but also shows that a rational assembly of zeolite crystals can greatly improve their adsorptive and/or catalytic properties.
These achievements have been published in Science (2022, 375, 29), Chem. Soc. Rev. (2019, 48, 885-907), ACS Appl. Mater. Interfaces (2021, 13, 58085-58095; 2017, 9 14899-14910), Chem. Commun. (2022, 58, 2041-2054), Catal. Sci. Technol. (2017, 7, 560-564) and other international journals.
This research was supported by the National Key R&D Program, the National Natural Science Foundation of China, and the Talent Innovation Program of Shanxi province.
© Institute of Coal Chemistry, Chinese Academy of Sciences, All Rights Reserved
Address: 27 South Taoyuan Road, Taiyuan, Shanxi, P.R.China
Tel: (86)351-4041627 Email: sxicc_en@sxicc.ac.cn