Recently, a research team led by Prof. Nanwen Li from the Institute of Coal Chemistry (ICC) of the Chinese Academy of Sciences (CAS) reported poly(oxindole biphenylene)-based ion-solvating membranes (ISMs) with highly stable oxindole/KOH complex ion pairs for use in water electrolysers. These ISMs exhibit promising alkaline stability at 80°C with a negligible conductivity decay over more than 15,000h and, thus, allow durable alkaline electrolysis over 2,500h, even at elevated temperatures and high operating voltages of 2.3V.
This work “An operationally broadened alkaline water electrolyser enabled by highly stable poly(oxindole biphenylene) ion-solvating membranes” was published in the Nature Energy on 15 January 2024.
Ion-solvating membranes (ISMs) are an alternative to proton-exchange and anion-exchange membranes for use in water electrolysers. ISMs do not have fixed ionic groups in their structure but instead gain their ionic conductivity through the uptake of liquid electrolyte. Although in principle they could offer improved stability over anion-exchange membranes due to the absence of easily degradable anion-exchange groups, stability gains have been modest. Here we report poly(oxindole biphenylene)-based ISMs with highly stable oxindole/KOH complex ion pairs for use in water electrolysers. These ISMs exhibit promising alkaline stability at 80°C with a negligible conductivity decay over more than 15,000h and, thus, allow durable alkaline electrolysis over 2,500h, even at elevated temperatures and high operating voltages of 2.3V. Moreover, they show ultralow gas permeation and, thus, low transient response times (<1s). They allow the use of non-precious-metal catalysts (Ni and Ni/Fe) and can be operated over a broad temperature range (35 to 120°C).
This work was supported by National Natural Science Foundation of China (Grant Nos. 21835005 and 22105217), the STS Project of the Chinese Academy of Sciences (Grant No. KFJ-STS-QYZD-2021-02-003) and the Natural Science Foundation of Shanxi Province (Grant No. 20210302124433).
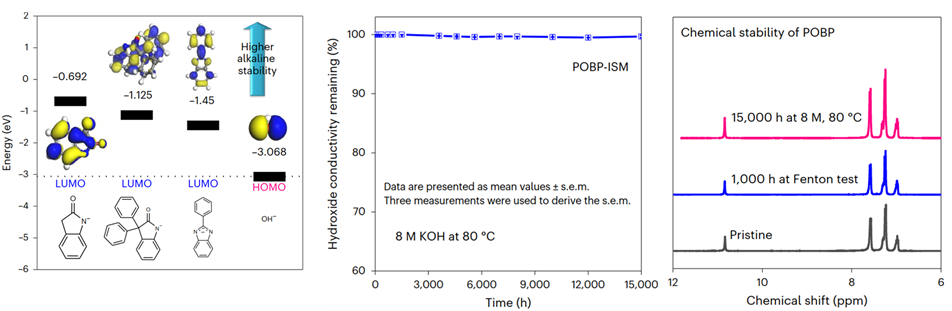
Figure 1. a) DFT calculation and alkaline stability of model compounds; b) the stability of conductivity; c) 1H NMR spectra of pristine and aged POBP after ex situ alkaline stability testing (15,000h at 8 M KOH and 80°C) and Fenton reagent testing (1000h at 80°C)
© Institute of Coal Chemistry, Chinese Academy of Sciences, All Rights Reserved
Address: 27 South Taoyuan Road, Taiyuan, Shanxi, P.R.China
Tel: (86)351-4041627 Email: sxicc_en@sxicc.ac.cn