Iron Oxide Clusters as Electron Donors Under Light Enhance Oxygen Reduction Kinetics at Atomically Dispersed Fe for Photocatalytic CH4 Partial Oxidation
Recently, a research conducted by Shuai Guo/Kuan Lu from the Institute of Coal Chemistry (ICC) of the Chinese Academy of Sciences (CAS), in collaboration with Prof. Pengfei Xie’s group from Zhejiang University and Prof. Sheng Dai’s group from East China University of Science and Technology found that the synergy between Fe2O3 nanocluster and Fe single atom establishes a dual-Fe site for O2 activation and subsequent CH4 partial oxidation. The light excitation of Fe2O3 nanoclusters with a relative narrow bandgap could deliver the electrons and protons to atomic Fe that facilitating the oxygen reduction kinetics for the robust of methanol synthesis.
This study entitled “Iron Oxide Clusters as Electron Donors Under Light Enhance Oxygen Reduction Kinetics at Atomically Dispersed Fe for Photocatalytic CH4 Partial Oxidation” was published in the Angewandte Chemie International Edition on 10 October 2024.
Photocatalytic CH4 oxidation to CH3OH emerges as a promising strategy to sustainably utilize natural gas and mitigate the greenhouse effect. However, there remains a significant challenge for the synthesis of methanol by using O2 at low temperature. Inspired by the catalytic structure in soluble methane monooxygenase (MMO) and the corresponding reaction mechanism, we prepared a biomimetic photocatalysts with the decoration of Fe2O3 nanocluster and satellite Fe single atom immobilized on carbon nitride. The catalyst demonstrates an excellent CH3OH productivity of 5.02 mmol·gcat-1·h-1 with methanol selectivity of 98.5%. Mechanism studies reveal that the synergy between Fe2O3 nanocluster and Fe single atom establishes a dual-Fe site as MMO for O2 activation and subsequent CH4 partial oxidation. Moreover, the light excitation of Fe2O3 nanoclusters with a relative narrow bandgap could deliver the electrons and protons to atomic Fe that facilitating the oxygen reduction kinetics for the robust of methanol synthesis.
This work was supported by National Key Research and Development Program of China, National Natural Science Foundation of China, Natural Science Foundation of Zhejiang Province etc.
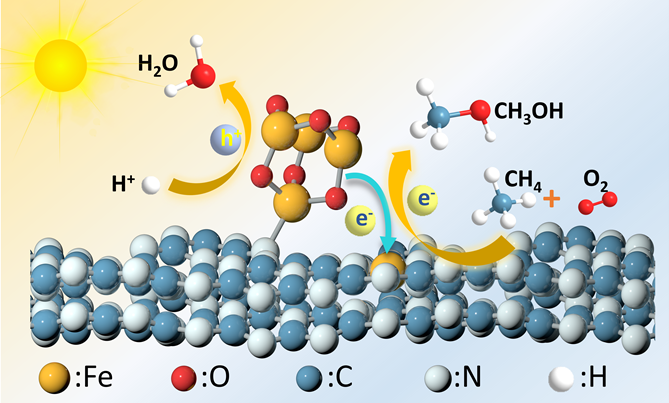
Figure 1. Fe2O3 nanoclusters provide electrons under the illumination to the satellite Fe single atom sites, promoting O2 activation and subsequent CH4 oxidation.
About the author:
Lu Kuan, working at Institute of Coal Chemistry, CAS, won the National Natural Science Foundation of China, Youth Innovation Promotion Association CAS, etc. He is committed to the development of in-situ dynamic simulations and the research on the relationship between catalyst structure and activity.
Email: lukuan@sxicc.ac.cn
Pengfei Xie is a researcher of the "Hundred Talents Program" of the School of Chemical Engineering and Biological Engineering, Zhejiang University, a doctoral supervisor. His main research interests are energy and environmental catalysis, focusing on the efficient conversion of small molecules in C1 and N1 gases.
Email: pfxie@zju.edu.cn
Dai Sheng, professor and doctoral supervisor of East China University of Science and Technology, is committed to explore the structural characteristics and dynamic evolution of environmental and energy catalysts at the atomic scale, analyze the active sites and reaction mechanisms of catalysts, and clarify the structure-activity relationship.
Email: shengdai@ecust.edu.cn
© Institute of Coal Chemistry, Chinese Academy of Sciences, All Rights Reserved
Address: 27 South Taoyuan Road, Taiyuan, Shanxi, P.R.China
Tel: (86)351-4041627 Email: sxicc_en@sxicc.ac.cn