Significant Progress in Exploring Novel Synthetic Route of Acrylic Acid Through Direct Conversion of Methanol and Acetic Acid
In recent years, new acrylic acid synthesis techniques based on non-petroleum routes have received extensive attention from both academia and industry. Among them, the aldol condensation route with acetic acid and formaldehyde as raw materials is the most representative. Formaldehyde is only available in low concentration aqueous solution (around 37%) in order to avoid its polymerization. When the aqueous solution is used as the raw material, water in the feed easily causes a series of problems, such as easy pulverization of the catalyst, high energy consumption for separation and purification and large amount of waste water.
To address these issues, a research team headed by Prof. Han Yizhuo, Prof. Zhang Qingde and Dr. Zhang Junfeng have made their efforts on development of a novel synthetic route using acetic acid and methanol as the reactants because of methanol’s easy availability, stability and safety.
The catalytic reaction is realized over a constructed TiO2-coated NASICON-type catalyst, on which methanol is dehydrogenated into formaldehyde first and then converted into acrylic acid(AA) and methyl acrylate (MA) via aldol condensation with acetic acid. The selectivity of the targeted AA+MA reached 56.1 %, corresponding to a spatiotemporal yield of 46.5 μmol·g-1·min-1 or 0.221 g·g-1·h-1 (Scheme 1). It is confirmed that the redox sites on the catalyst surface were mainly from the TiO2 coating, while the acid-base sites required for aldol condensation were from the NASICON substrate material. With efficient synergy of redox and acid-base properties, the direct synthesis of acrylic acid and methyl acrylate has been realized successfully. By correlating the characterization results and reaction properties, a possible reaction pathways for acrylic acid synthesis was also proposed (Figure 1). This study provides an alternative strategy for the design of efficient catalyst for similar cascade reaction.

Scheme 1. Direct synthesis of acrylic acid from methanol and acetic acid on TiO2-coated NASICON
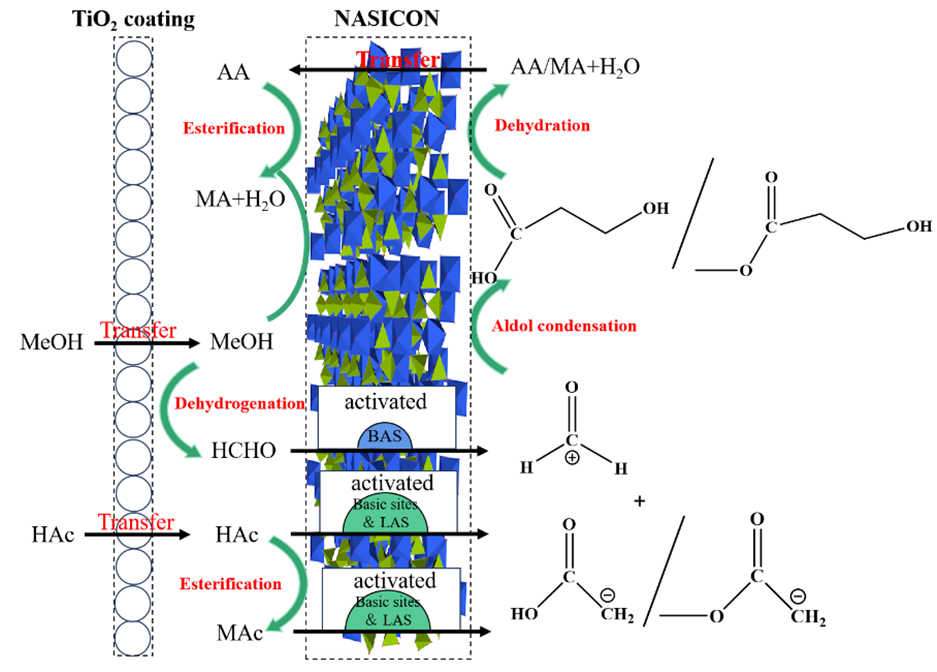
Figure 1. Proposed catalytic reaction mechanism of AA and MA formation on the bi-functional TiO2-coated NASICON catalyst
This work has been published in the Journal of Catalysis on 17 June 2024 with the title of “Direct synthesis of acrylic acid from methanol and acetic acid over a constructed TiO2-coated NASICON catalyst”. The project is funded by the Natural Science Foundation of Shanxi Province (NO.20210302123010) and the Innovation Foundation of ICC, CAS (No. SCJC-DT-2023-05).
About the author:
Zhang Junfeng, associate professor, State Key Laboratory of Coal Conversion (SKLCC), Institute of Coal Chemistry, Chinese Academy of Sciences. zhangjf@sxicc.ac.cn
Zhang Qingde, professor, State Key Laboratory of Coal Conversion (SKLCC), Institute of Coal Chemistry, Chinese Academy of Sciences. qdzhang@sxicc.ac.cn
Han Yizhuo, professor, State Key Laboratory of Coal Conversion (SKLCC), Institute of Coal Chemistry, Chinese Academy of Sciences. hanyz@sxicc.ac.cn
© Institute of Coal Chemistry, Chinese Academy of Sciences, All Rights Reserved
Address: 27 South Taoyuan Road, Taiyuan, Shanxi, P.R.China
Tel: (86)351-4041627 Email: sxicc_en@sxicc.ac.cn