Operando mobile catalysis for reverse water gas shift reaction
Recently, a research group led by Prof. Bin Zhang and Yong Qin from the Institute of Coal Chemistry (ICC) of the Chinese Academy of Sciences (CAS), in collaboration with/cooperating with Dr. Xinchen Liu, proposed a new concept of Operando mobile catalysis. This work entitled “Operando mobile catalysis for reverse water gas shift reaction” was published in the Angewandte Chemie International Edition on March 18, 2024.
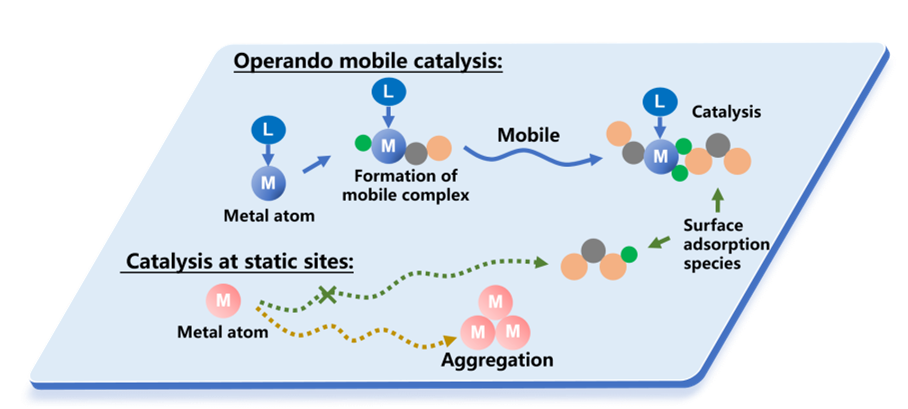
Metal atoms on the support serve as active sites for many heterogeneous catalysts. However, the active metal sites on the support are conventionally described as static, and the intermediates adsorbed on the support far away from the active metal sites cannot be transformed. In this work, the authors report the first example of operando mobile catalysis to promote catalytic efficiency by enhancing the collision probability between active sites and reactants or reaction intermediates. Specifically, ligand-coordinated Pt single atoms (isolated MeCpPt- species) are bonded on CeO2 and transformed into mobile MeCpPt(H)CO complexes during the reverse water gas shift reaction for operando mobile catalysis. This strategy enables the conversion of inert carbonate intermediates on the CeO2 support and a turnover frequency (TOF) dramatically higher than those of Pt catalysts reported in the literature. Operando mobile catalysis presents a promising strategy for designing high-efficiency heterogeneous catalysts for various chemical reactions and applications.
To achieve both stability and long-distance mobility of metal atoms on the support, the authors propose an operando mobile catalysis strategy. Initially, isolated metal species are bonded to the support, and they are then reversibly transformed into mobile isolated metal species for catalysis during the reaction.
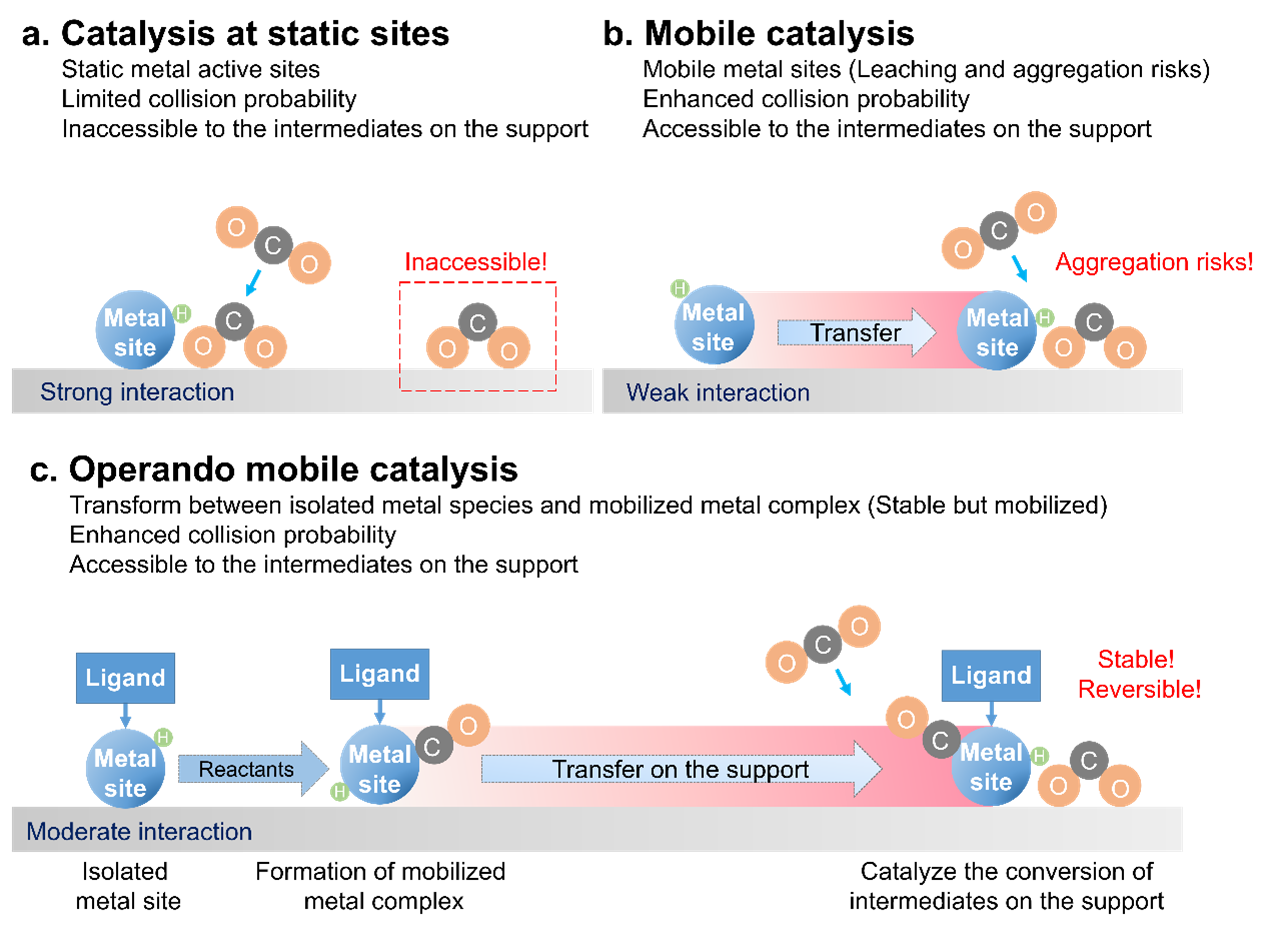
Figure 1. Schematic diagrams illustrating the processes of catalysis at static sites.
Atomically dispersed isolated Pt species coordinating with a methylcyclopentadiene ligand (MeCp) were bonded on CeO2 nanorods using a Pt organic precursor (MeCpPtMe3) via the half-reaction of atomic layer deposition. The MeCpPt/CeO2 showed exceptionally high performance, yielding a TOF of 6358 mol CO2 molPt-1·h-1 at 300 °C, 3~20-fold higher than these reported Pt catalysts under similar reaction conditions. When the Pt loading (or density of Pt atoms on CeO2) increased, the TOF over x-MeCpPt/CeO2 gradually decreased.

Figure 2.Structural characterization of isolated Pt species on MeCpPt/CeO2.
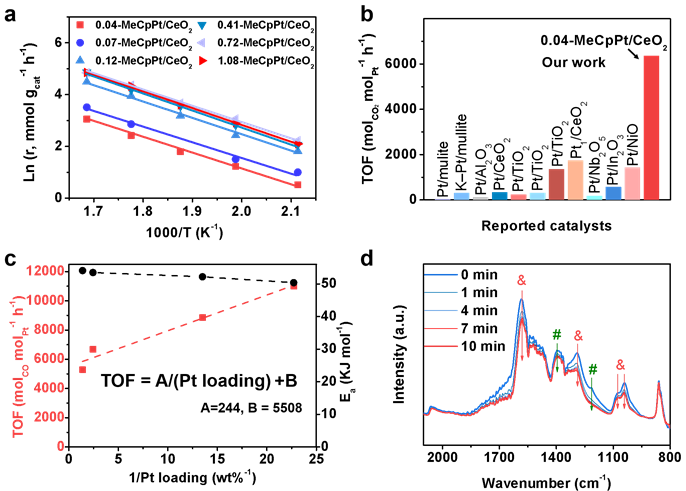
Figure 3. Evaluation of RWGS activity dynamic study.
The catalytic mechanism and structural evolution of the catalyst during the reaction were observed by quasi-in-situ XPS, in-situ XAFS, and in-situ DRIFTS. These results demonstrate that mobilizable isolated Pt species with low Pt-O coordination arise by reducing the Pt-O-Ce bond and forming the Pt-H bond during the reaction.
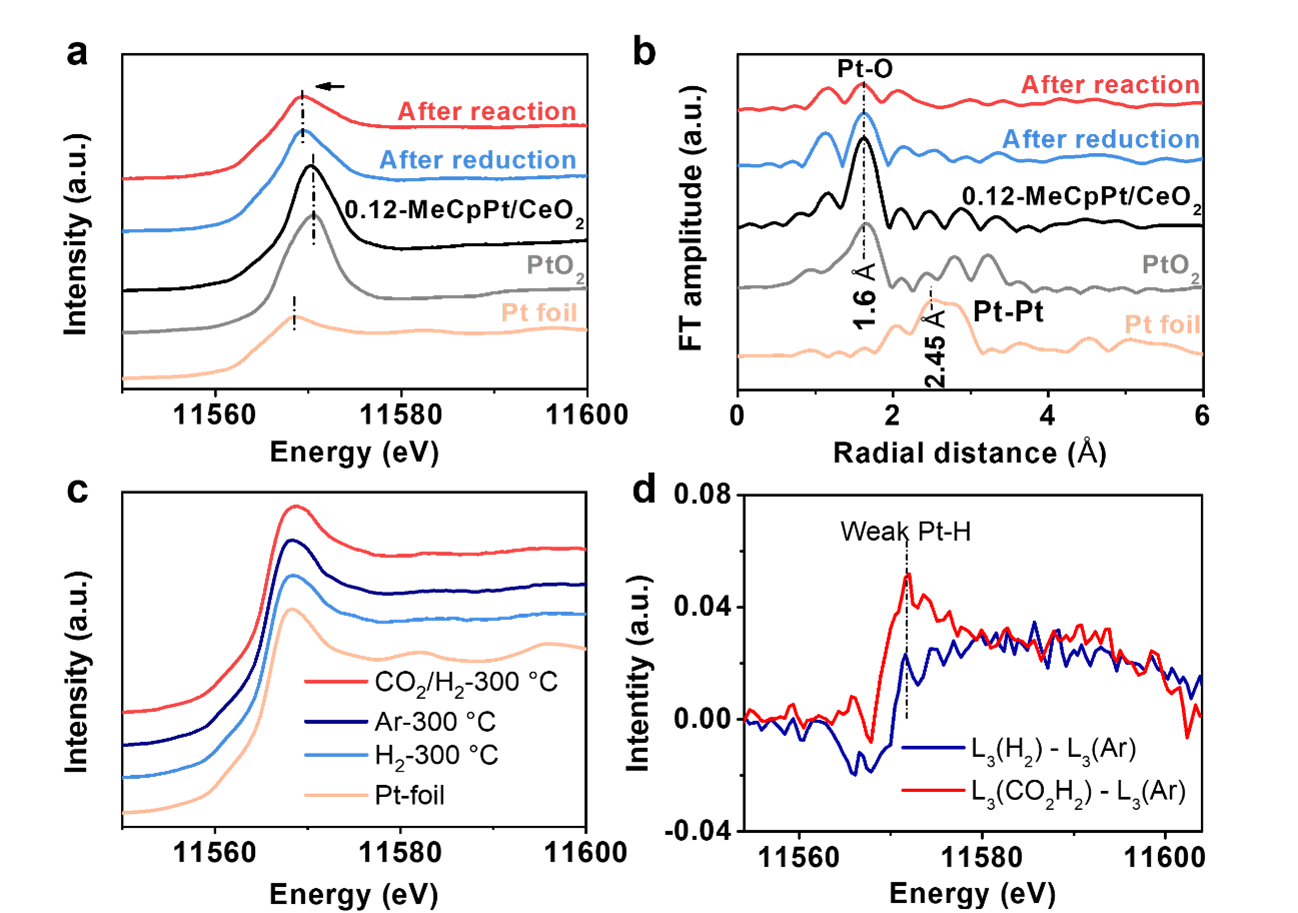
Figure 4. Structure evolution of mobile Pt species.
When the authors mixed MeCpPt/CeO2 with TiO2 nanoparticles and tested its CO2 hydrogenation performance, the Pt single atoms migrated from the CeO2 nanorod to TiO2 nanoparticles. The migration of Pt single atoms between particles indicates the presence of mobile Pt species during the reaction, which is pivotal for elucidating the operando mobile catalysis mechanism.
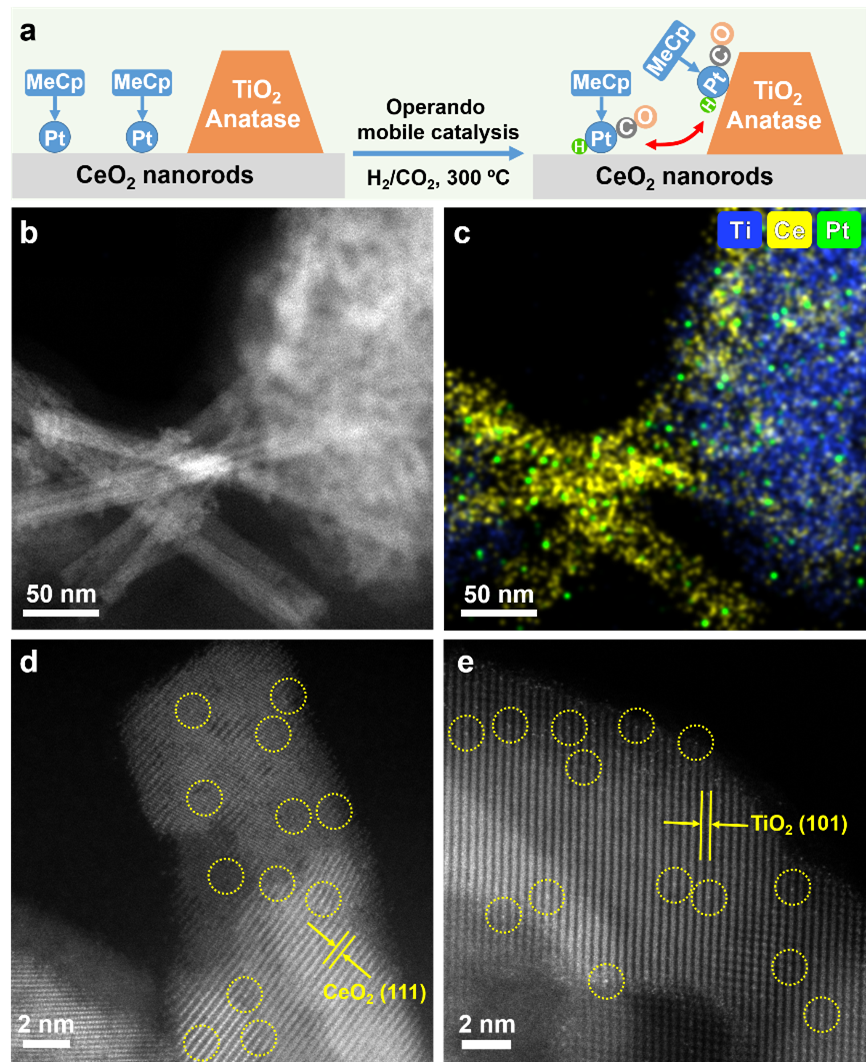
Figure 5. Operando mobile catalysis behavior across nanoparticles.
DFT and ab initio molecular dynamics simulation suggested that the MeCpPt complexes coordinated with H and CO to produce mobile MeCpPt(H)CO complex, which can migrate along the surface of CeO2 without leaching and aggregating and move to carbonate intermediates to transform them.
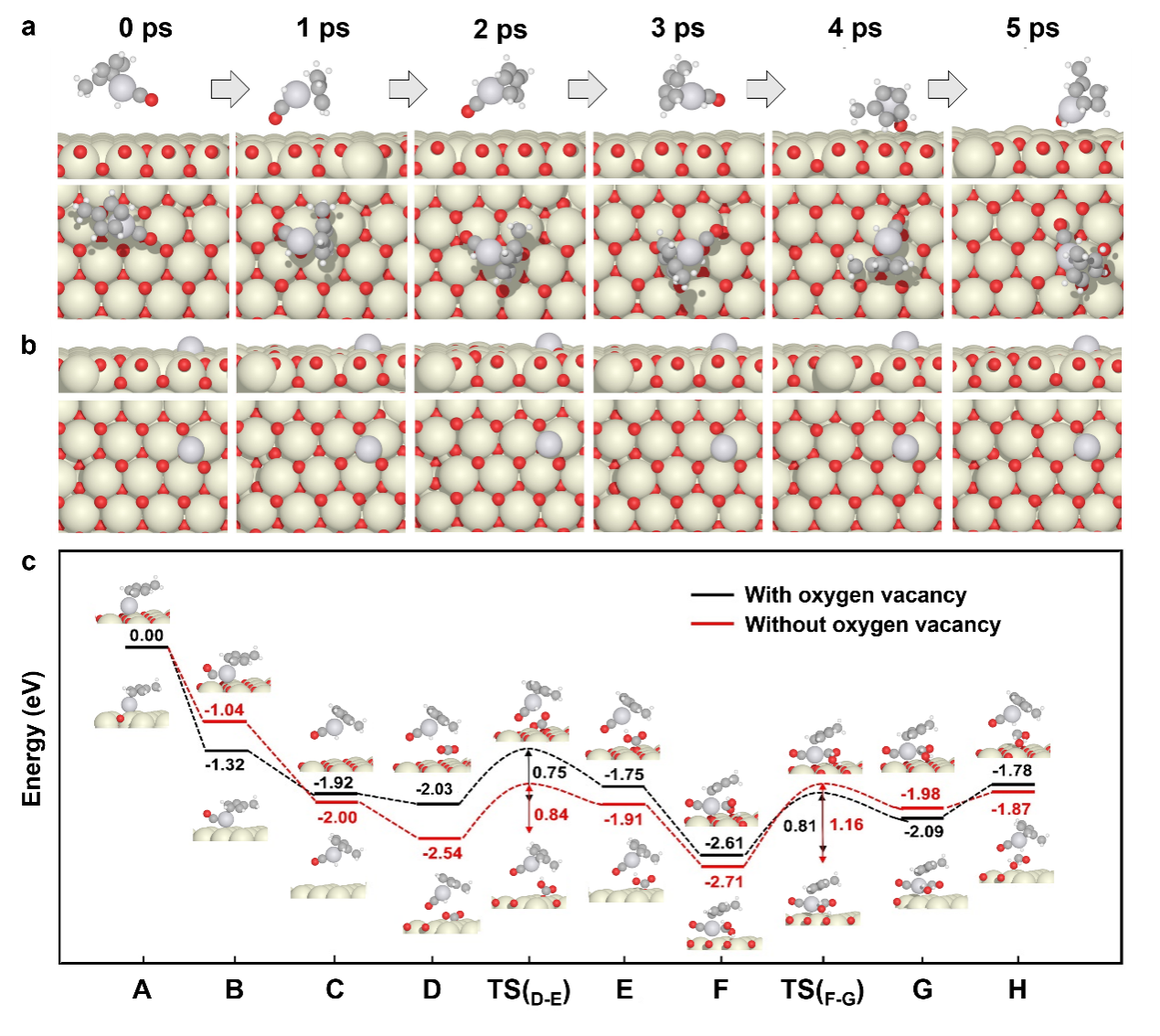
Figure 6. Mobile catalysis mechanism.
Operando mobile catalysis presents an opportunity to design high-efficiency heterogeneous catalysts that efficiently transform stable adsorbed intermediates on the support. Further research in this area can pave the way for developing sustainable and efficient catalytic processes for various chemical reactions and applications.
H. Liang, B. Zhang, M. Hong, X. Yang, L. Zhu, X. Liu, Y. Qi, S. Zhao, G. Wang, A. P. van Bavel, X. Wen, Y. Qin, Angew. Chem. Int. Ed. 2024, 63, e202318747. https://doi.org/10.1002/anie.202318747
© Institute of Coal Chemistry, Chinese Academy of Sciences, All Rights Reserved
Address: 27 South Taoyuan Road, Taiyuan, Shanxi, P.R.China
Tel: (86)351-4041627 Email: sxicc_en@sxicc.ac.cn