ICC CAS has made significant progress in the theory of phase transition regulation of iron-based catalysts for Fischer-Tropsch synthesis
Recently, the team led by Researcher Wen Xiaodong and Liu Xingchen from the the Institute of Coal Chemistry (ICC) of the Chinese Academy of Sciences (CAS), has made significant progress in the theory of phase transition regulation of catalysts and the controllable synthesis of iron-based catalysts. This research result, titled "Rational Synthesis of Iron Carbide Nanocatalysts for Fischer-Tropsch Synthesis: The Role of Carbon Chemical Potential", was published in the top international Journal in the field of chemistry "Journal of the American Chemical Society".
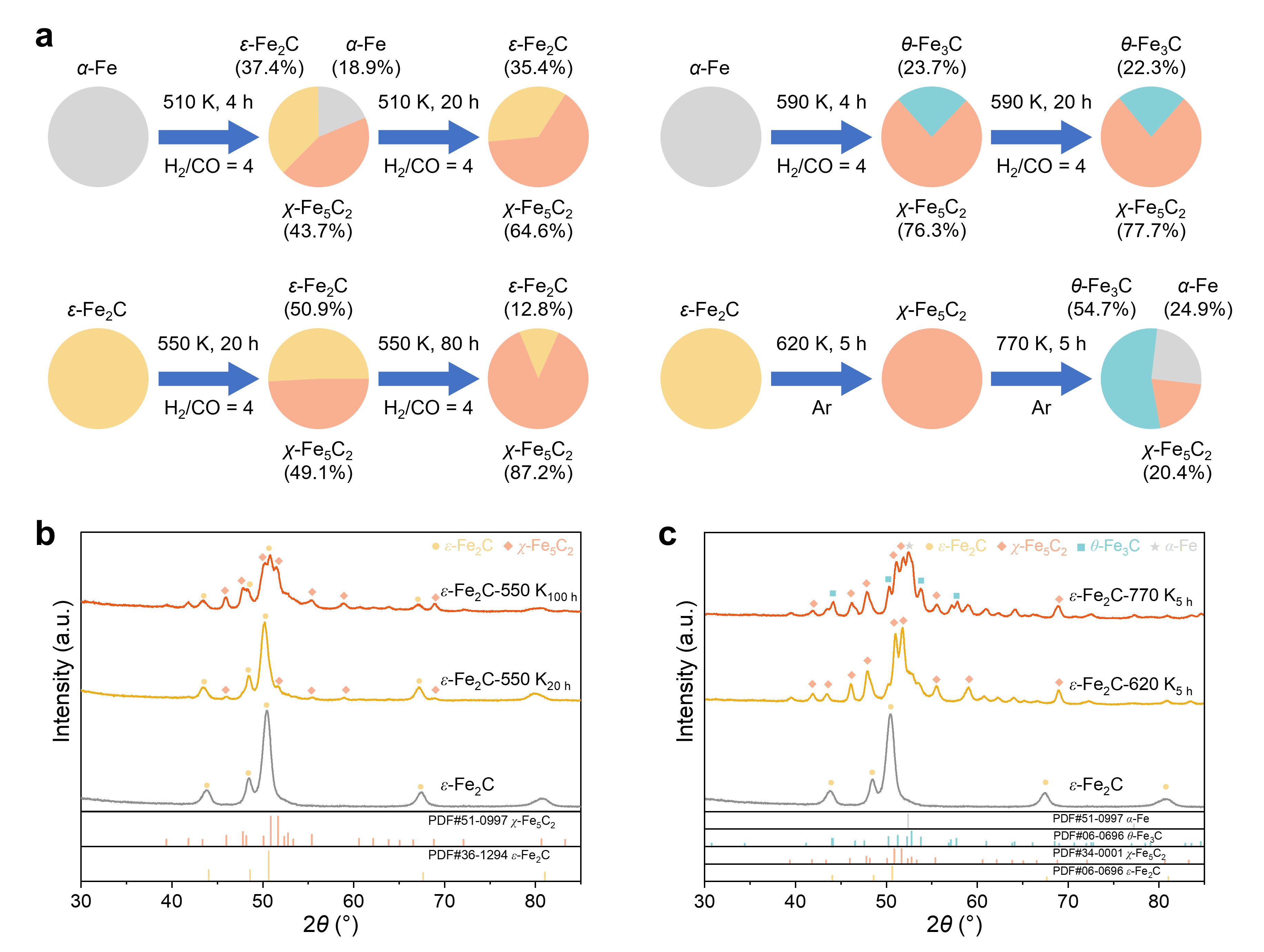
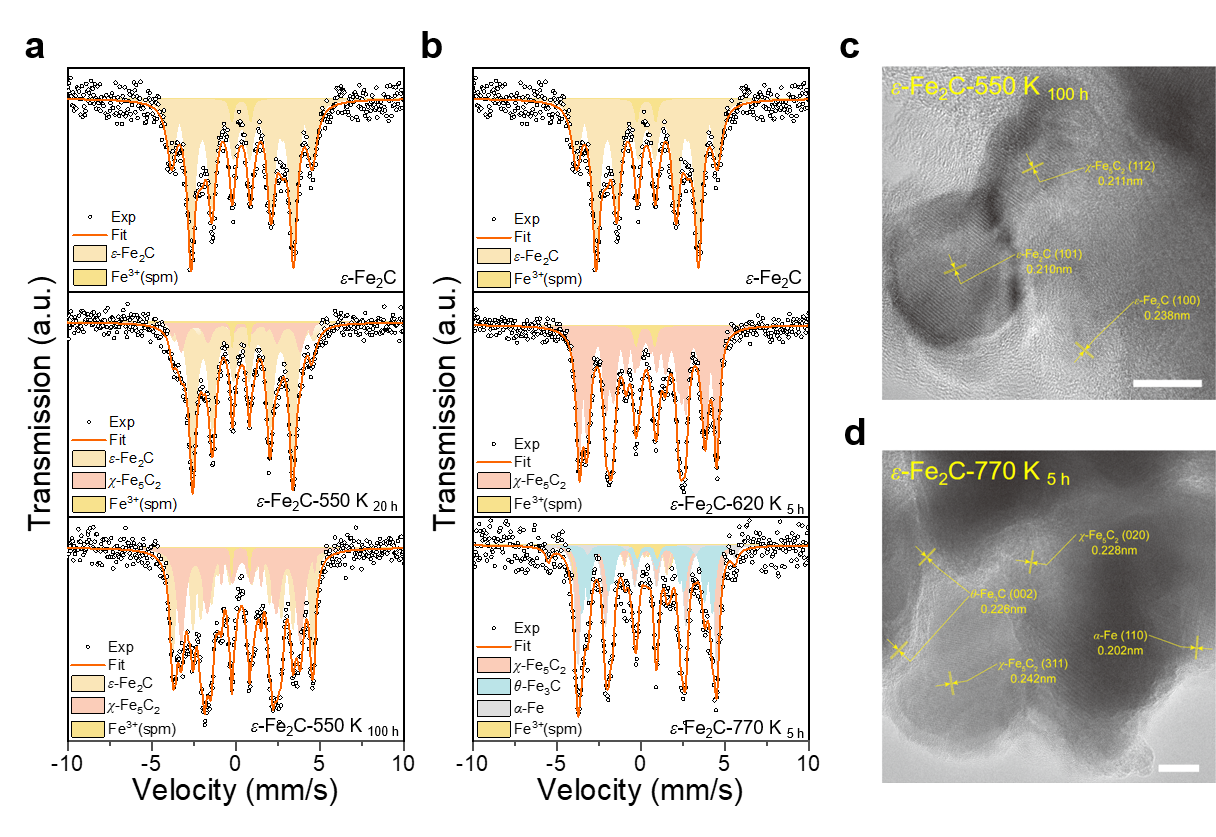
ABSTRACT: Understanding nanoparticle phase behavior under reactive conditions is critical for rational catalyst design in sustainable energy technologies. Yet, the phase transformations of multielement catalytic solids remain poorly understood, particularly when accompanied by compositional changes in reactive environments. Using iron carbide (FexCy) as a model system, we investigate size-dependent phase stability and transformation kinetics across various chemical environments relevant to Fischer−Tropsch synthesis. By integrating nanoscale thermodynamics and kinetics into a unified theoretical framework, we construct comprehensive phase diagrams that explain the anomalous predominance of the χ-Fe5C2 phase and the rarity of the Fe7C3 phase-a longstanding puzzle in heterogeneous catalysis. Located at the intersection of all other phase stability regions across a broad range of carbon chemical potentials and particle sizes, χ-Fe5C2 functions as a central hub, facilitating transitions among various iron carbide phases, and has relatively low formation kinetic barriers from neighboring phases. In contrast, Fe7C3 is thermodynamically stable and kinetically favorable only within a very narrow range (approximately 0.03 eV) of carbon chemical potential at relatively large particle sizes. Our theoretical predictions, rigorously validated through precisely designed phase transformation experiments, reveal how nanoscale effects fundamentally govern phase selectivity and transformation pathways in iron carbides. This integrated approach provides mechanistic insights into the in situ formation and interconversion of catalytically active phases and establishes a general framework for predicting and manipulating phase behavior in complex multielement nanocatalysts for carbon-neutral fuel production and environmental applications.
Huan Ma, Yi Cai, Yueyue Jiao, Xingwu Liu, Jinjia Liu, Wenping Guo, Xingchen Liu, Yongwang Li, and Xiaodong Wen, Journal of the American Chemical Society 2025 147 (45), 41452-41461, DOI: 10.1021/jacs.5c11223
© Institute of Coal Chemistry, Chinese Academy of Sciences, All Rights Reserved
Address: 27 South Taoyuan Road, Taiyuan, Shanxi, P.R.China
Tel: (86)351-4041627 Email: sxicc_en@sxicc.ac.cn