|
|
| A new mechanism for carbon nanotube evolution |
 |
Text Size: A A A |
|
|
|
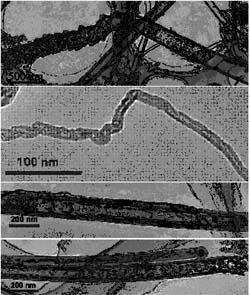 |
| Fig 1. The morphologies of representative intermediates for the nanotubes evolution. | | Key discoveries on the growth mechanism of carbon nanotubes (CNTs) have recently been achieved by CAS researcher ZHU Zhenping and his research group at the State Key Laboratory of Coal Conversion, the Institute of Coal Chemistry of CAS under the sponsorship of National Natural Science Foundation of China and CAS Bairen Program.
CNTs, namely molecular-scale tubes of graphitic carbon, are among the stiffest and strongest fibers ever known with remarkable electronic properties and many other unique characteristics. They have attracted huge academic and industrial interest, with thousands of papers on them being published every year.
However, the fast reaction feature of the CNT synthesis or growth and the requirement of high temperature environment, means a headache for scientists to look into its evolution mechanism, while the widely accepted theory in this field proposed by R. T. K. Baker in the 1970s, known as the "absorption-diffusion-graphitization model," has proved weak in explaining many experimental phenomena and facts.
Zhu and his colleagues successfully froze the synthetic reaction at intermediary stages to observe the detailed morphologies and structures of the intermediates obtained (see Figure 1), and a kindred evolution linkage was unveiled among carbon nanoparticles, nanowires, and nanotubes in the vapor-catalyst-involved synthetic processes (see Figure 2): Tiny carbon nanoparticles, formed from a condensation of gaseous carbon species, were self-assembled into nanowires driven by an anisotropic interaction, which then developed into nanotubes as a consequence of particle coalescence and structural crystallization. The function of metal catalysts is to promote the anisotropic interactions of nanoparticles to grow into nanowires and the structural crystallization of nanowires into nanotubes. Meanwhile, the scientists also achieved an annealing transformation of carbon nanoparticles into nanotubes, providing further evidence for the newly discovered evolution mechanism.
The above research results, published in the Nov. 10th issue (2006) of theJournal of the American Chemical Society(JACS), will hopefully give theoretical guidance to the technological development of CNTs synthesis and structural management.
Zhu and his team in 2005 proposed and experimentally validated a new concept in catalytic CNT growth: self-catalytic behavior of CNTs, which provides a better understanding of the growth mechanism of carbon nanotubes in a metal catalytic process. This result was published in the October 20th issue ofJACS.
 |
| Figure 2. Schematic diagram of the proposed particle-wire-tube mechanism for nanotube evolution. |
| |
|
|
